
Use the solubility rules to predict if each double-replacement reaction will occur and, if so, write a balanced chemical equation.Assuming that each double-replacement reaction occurs, predict the products and write each balanced chemical equation.Li + H 2O → ? (Hint: treat H 2O as if it were composed of H + and OH − ions.).Use the periodic table or the activity series to predict if each single-replacement reaction will occur and, if so, write a balanced chemical equation.Assuming that each single-replacement reaction occurs, predict the products and write each balanced chemical equation.What are the general characteristics that help you recognize double-replacement reactions?.What are the general characteristics that help you recognize single-replacement reactions?.reactants: nitrogen and hydrogen product: ammoniaĤ.3: Types of Chemical Reactions - Single and Double Displacement Reactions.How would you write the balanced chemical equation in Exercise 12 if all the substances except water were gases and water itself were a liquid?.How would you write the balanced chemical equation in Exercise 10 if all substances were gases?

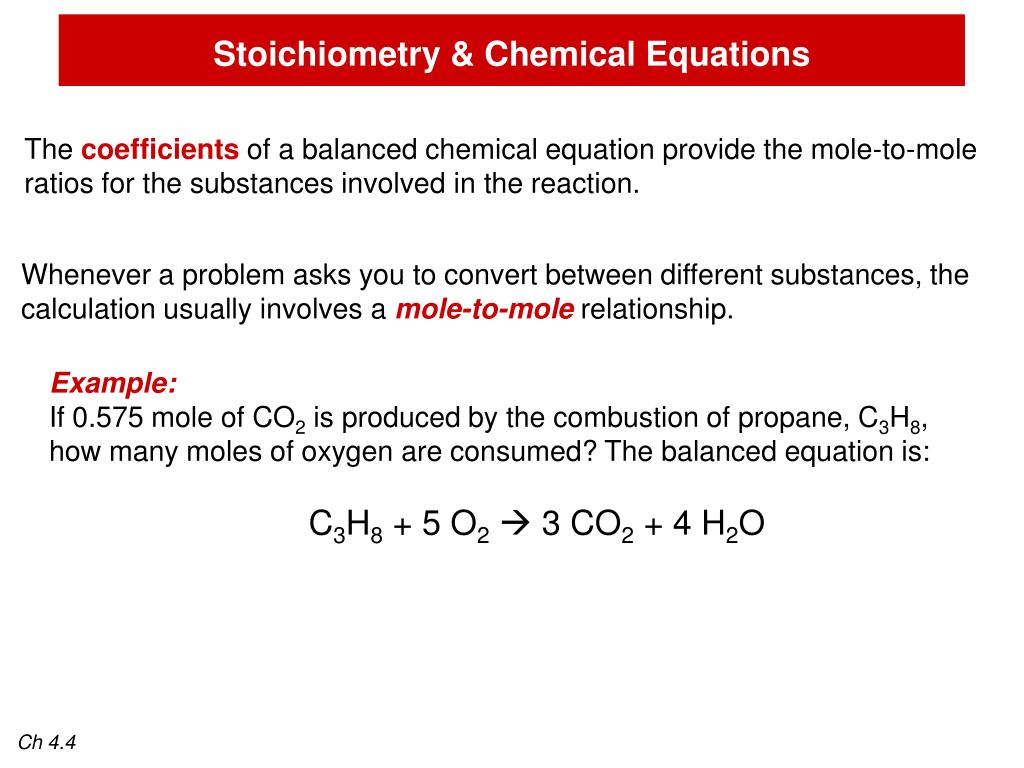
The formula for propane is C 3H 8.īalance: _C 2H 4 + _O 2 → _CO 2 + _H 2O

Write and balance the chemical equation described by Exercise 4. Write and balance the chemical equation described by Exercise 3. Write and balance the chemical equation described by Exercise 2. Write and balance the chemical equation described by Exercise 1. From the statement “nitrogen and hydrogen react to produce ammonia,” identify the reactants and the products.įrom the statement “sodium metal reacts with water to produce sodium hydroxide and hydrogen,” identify the reactants and the products.įrom the statement “magnesium hydroxide reacts with nitric acid to produce magnesium nitrate and water,” identify the reactants and the products.įrom the statement “propane reacts with oxygen to produce carbon dioxide and water,” identify the reactants and the products.


 0 kommentar(er)
0 kommentar(er)
